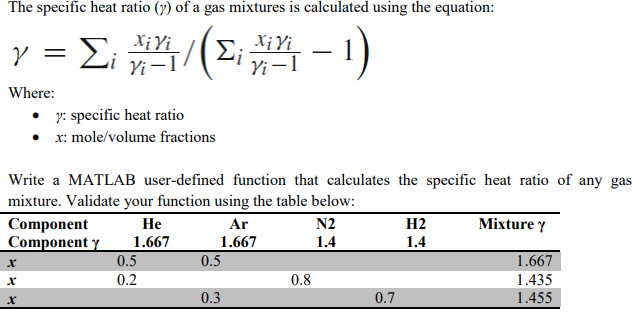
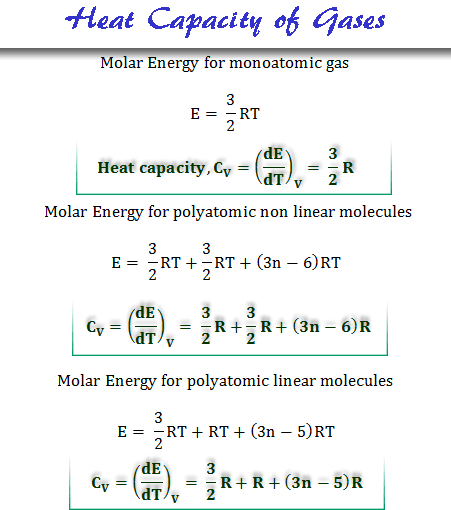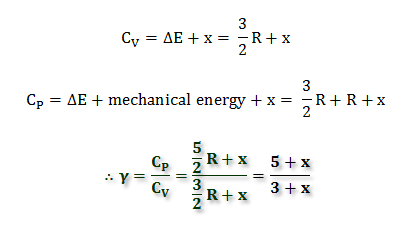Percentage Deviations of Isobaric Heat Capacities Calculated for Dry... | Download Scientific Diagram

The molar specific heat at constant volume of gas mixture is `(13R)/(6)`. The gas mixture consis... - YouTube

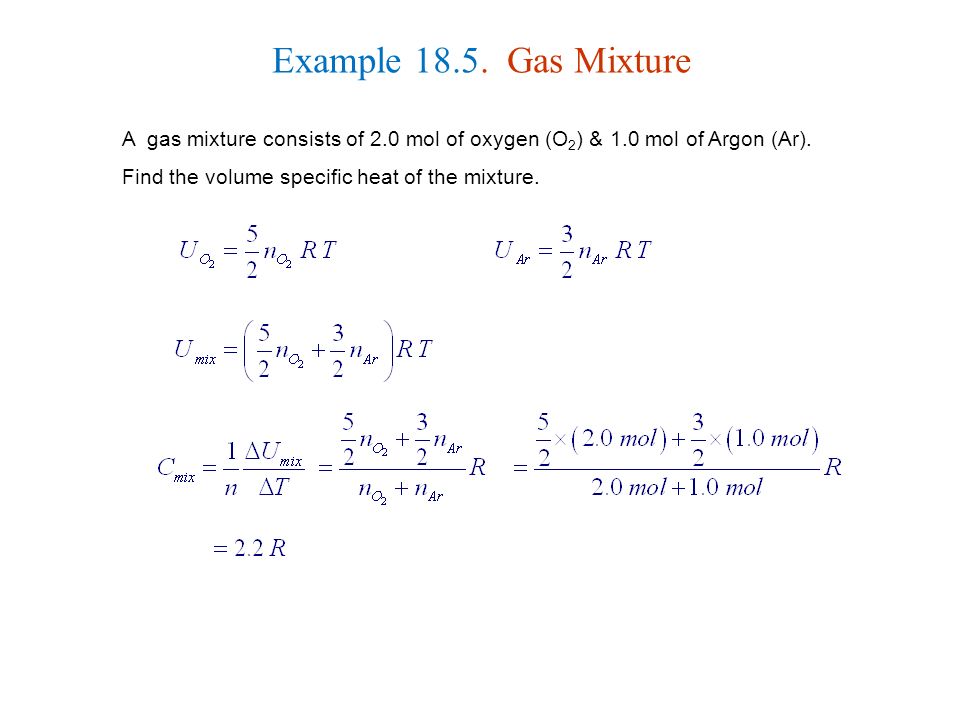
Calculate the value of gamma = Cp / Cv for a gaseous mixture consisting of v1 = 2.0 moles of oxygen and v2 = 3.0 moles of carbon dioxide. The gases are assumed to be ideal.
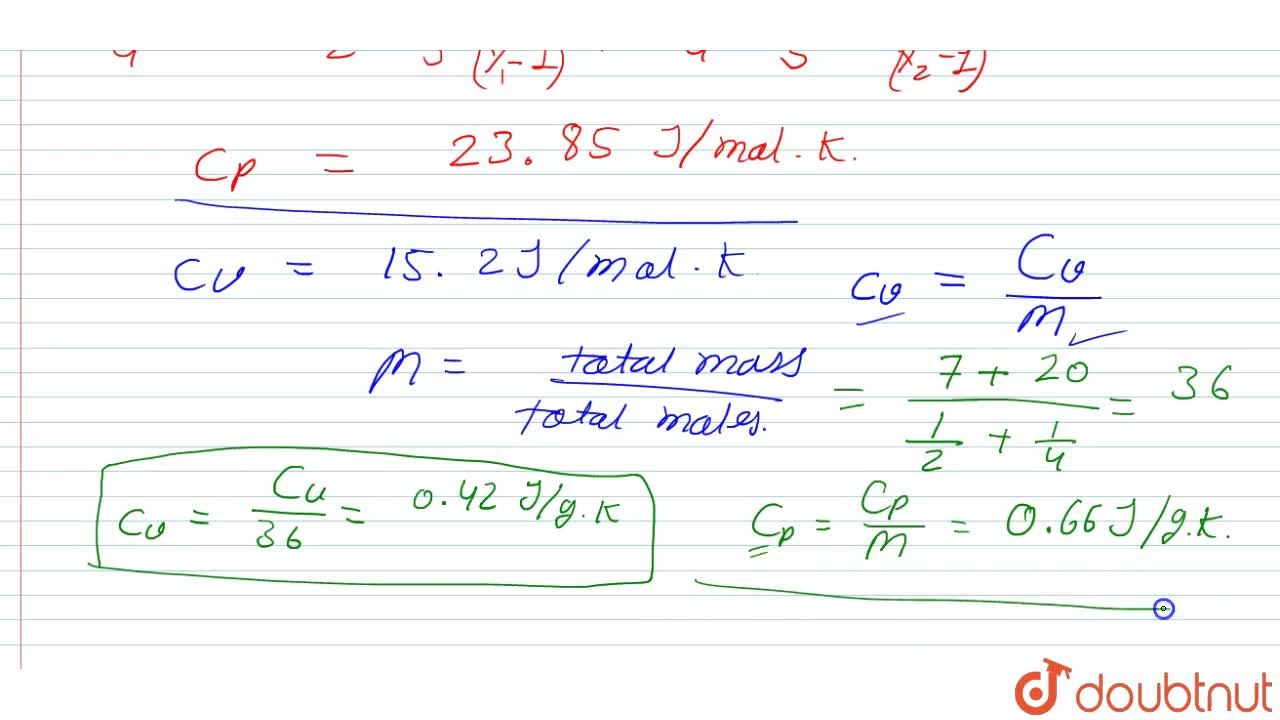
Find the specific heat capacities cv and cp for a gaseous mixture consisting of 7.0 g of nitrogen and 20 g of argon. The gases are assumed to be ideal.

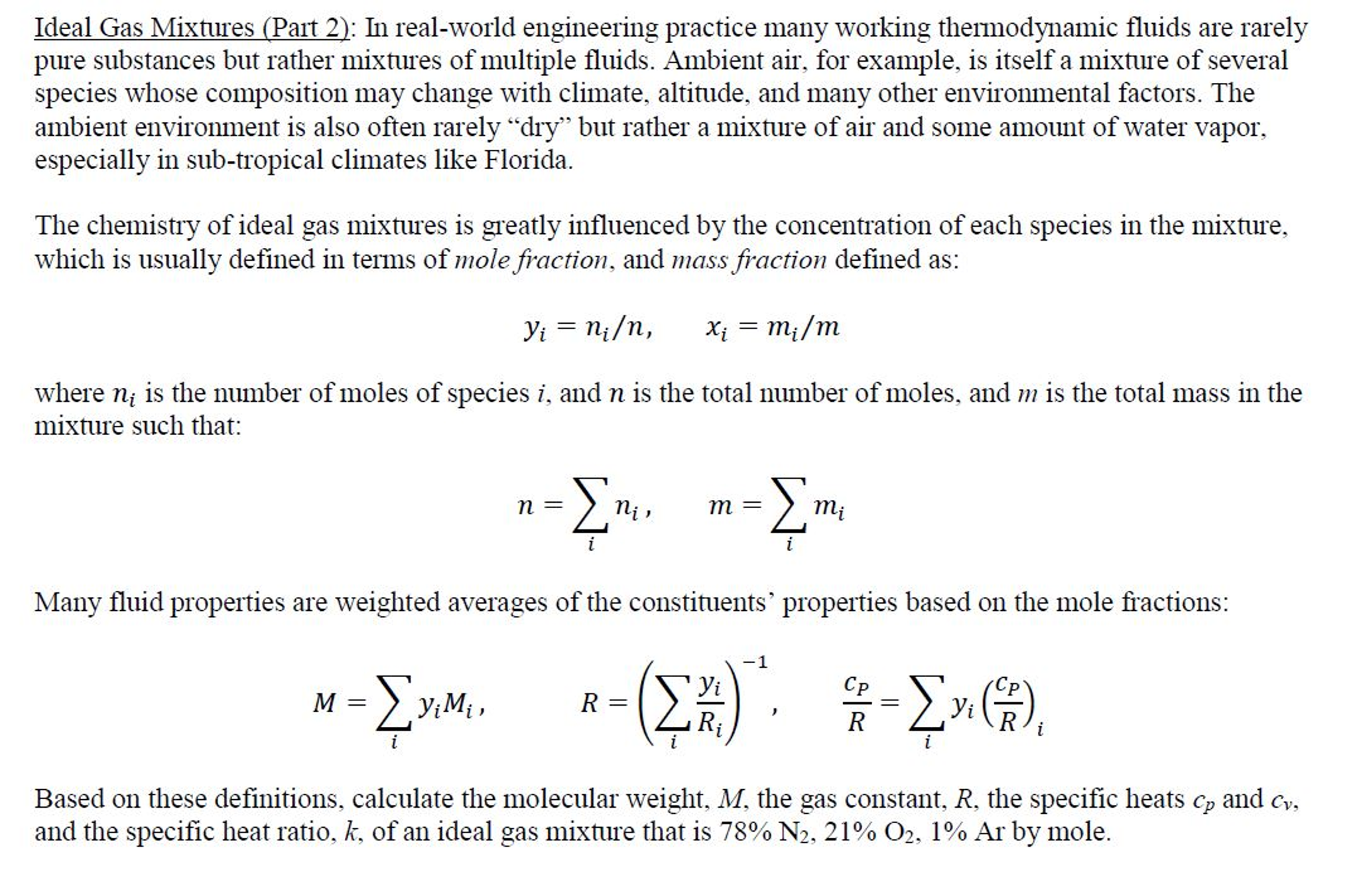
How can I find Cp_(T) (specific heat at constant pressure) of mixture gas for PEM Fuel Cell Modeling ?
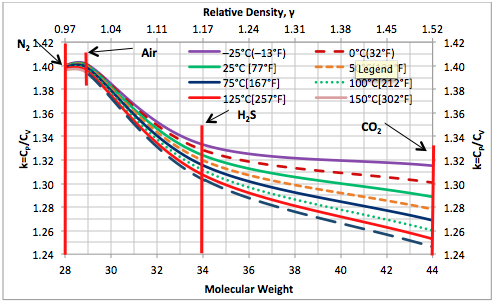
Variation of Ideal Gas Heat Capacity Ratio with Temperature and Relative Density | Campbell Tip of the Month

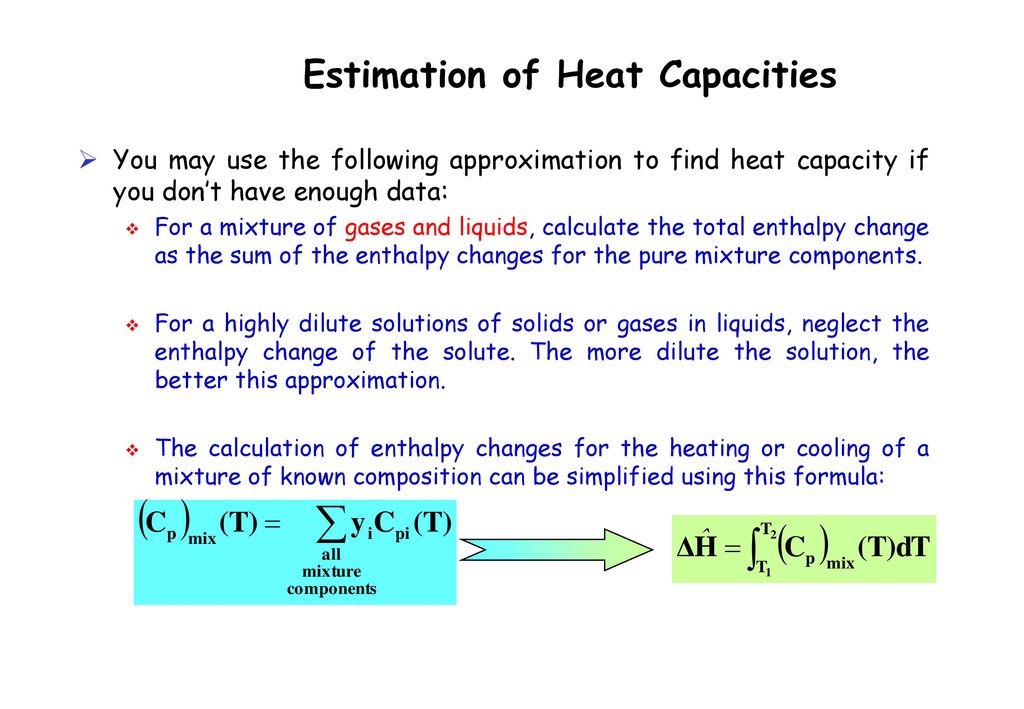
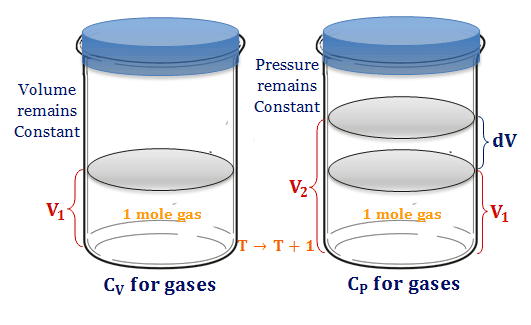
CHAPTER 4 HEAT EFFECT. Consider the process of manufacturing ETHYLENE GLYCOL (an antifreeze agent) from ethylene : -Vaporization -Heating Ethylene (liquid) - ppt download