Sublimation Bundle Starter Package with heat and mug press, Sublimation Bundle Starter Package with heat and mug pressSublimation Printer, heat press, mug press bundleThis bundle is a perfect way to get into

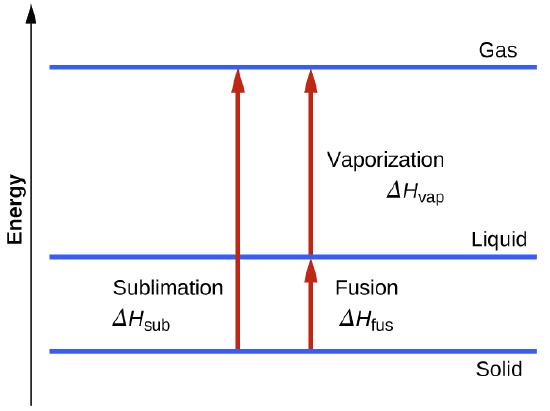
Correct relationship between heat of fusion Δ Hfus , heat of vaporization Δ Hvap and heat of sublimation Δ Hsub is:

Given: The heat of sublimation of K(s) is 89 kJ mol^(-1). K(g) rarrK^(o+)(g)+e^(-), DeltaH^(Theta) = 419 kJ F(2)(g) rarr 2F(g),DeltaH^(Theta) = 155 kJ The lattice enegry of KF(s) is -813kJ mol^(-1), the

Enthalpy of sublimation of iodine is 24 cal g^-1 at 200^oC . If specific heat of I2(s) and I2 (vap) are 0.055 and 0.031 cal g^-1 K^-1 respectively, then enthalpy of sublimation

Amazon.com: Sublimation Blanks White Coasters,20 Pcs Blank Round Soft Bar Coasters,4 Inch Heat Press Sublimation Coaster for Company Family Crafts Painting Image Logo : Everything Else

Enthalpy of sublimation of `I_(2)(s)` at `200^(@)C` is 24J/gm and specific heat of `I_(2)` (vapor) - YouTube