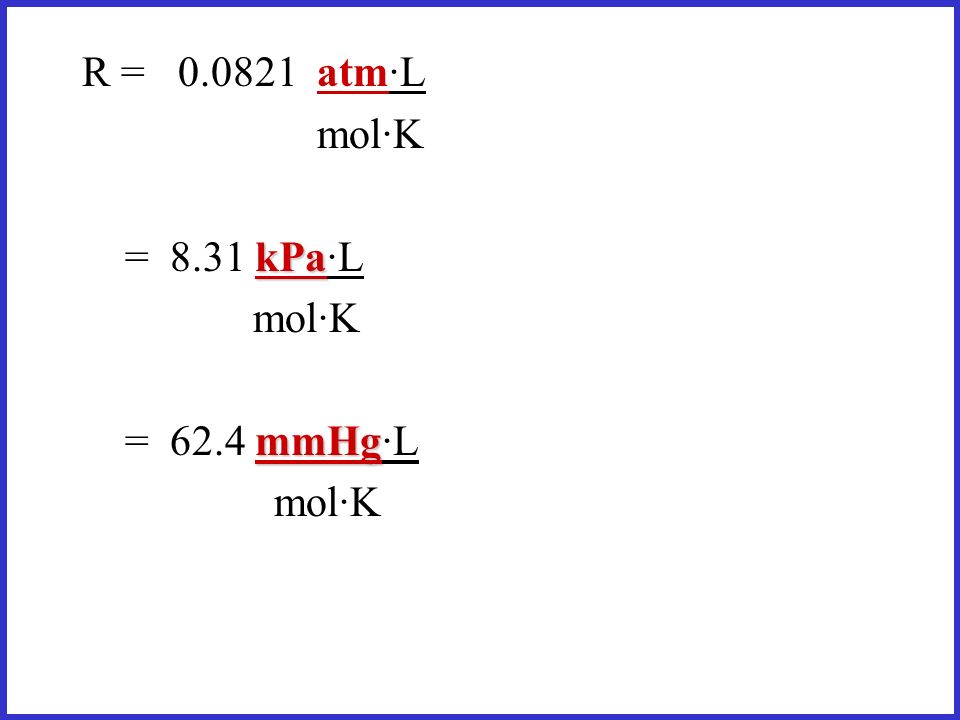
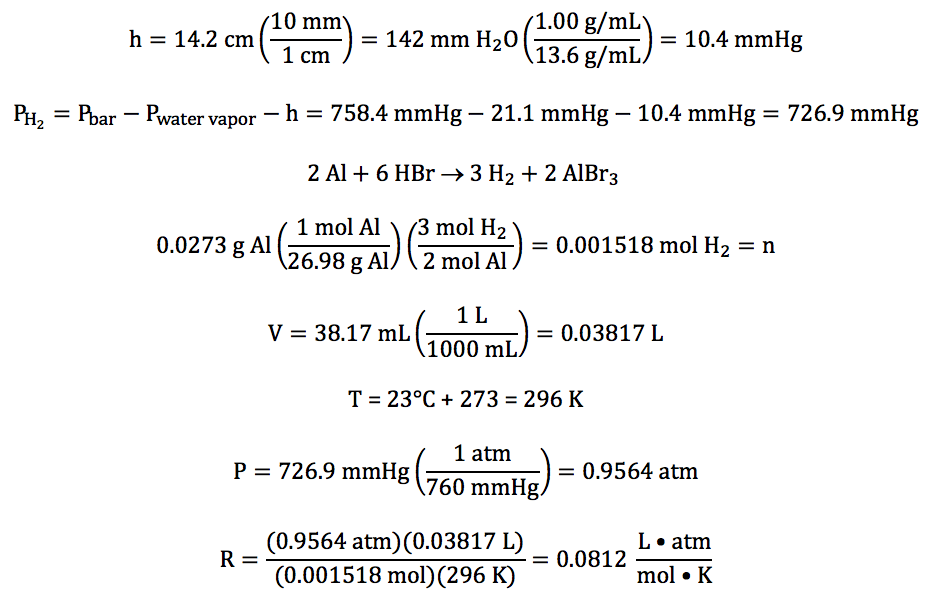
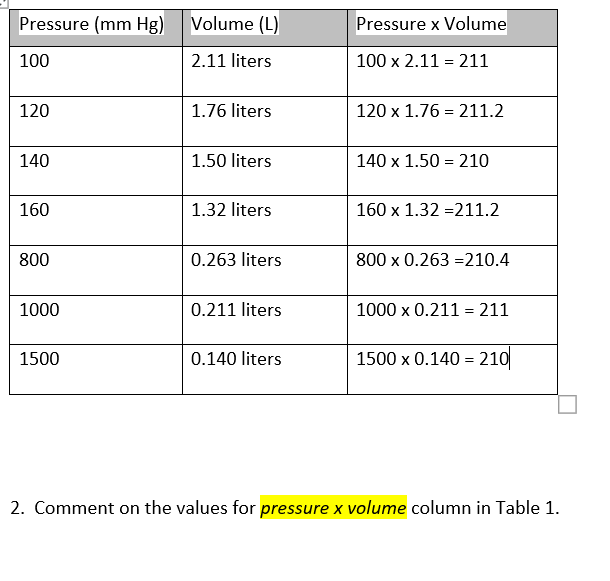
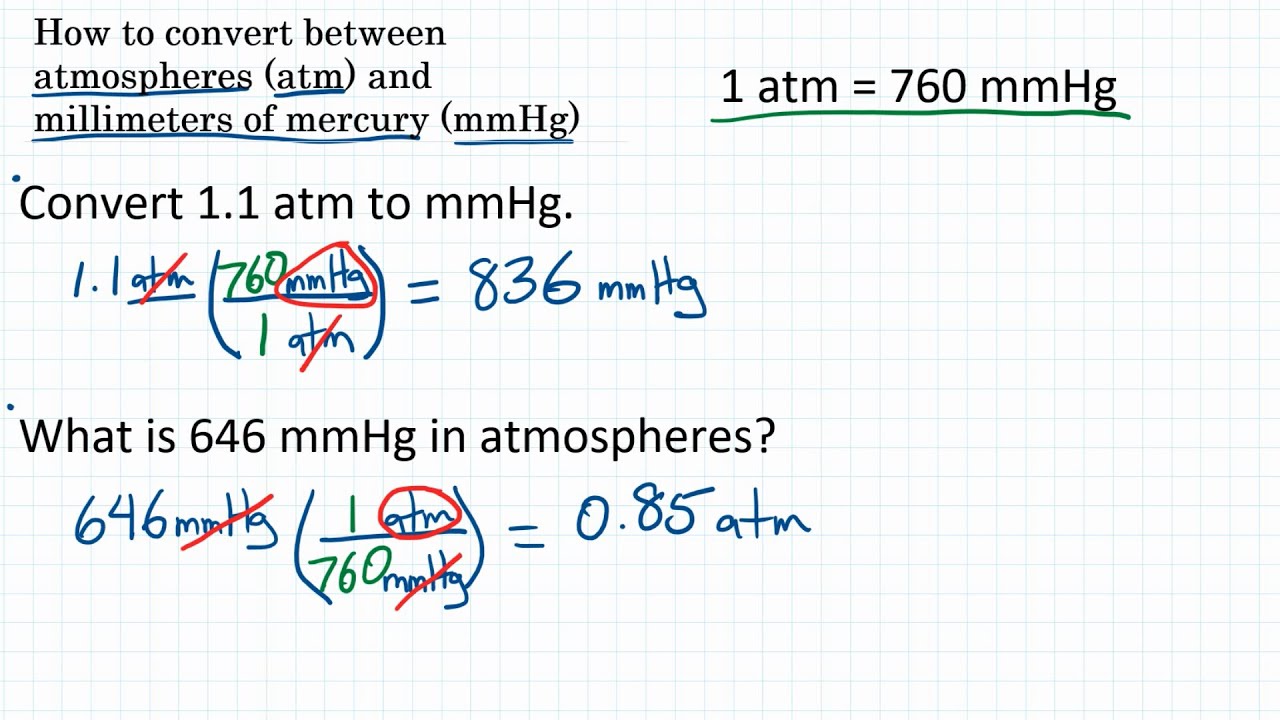
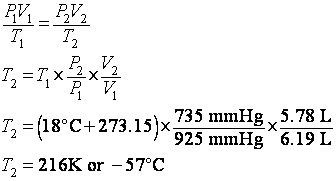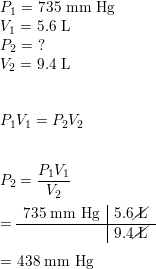Warm up Convert 65.0 mmHg to atm Reference: 1 atm = 760 mmHg = kPa 2. A helium balloon has a volume of 2.75 L at 20 ºC. the volume decreases. - ppt download
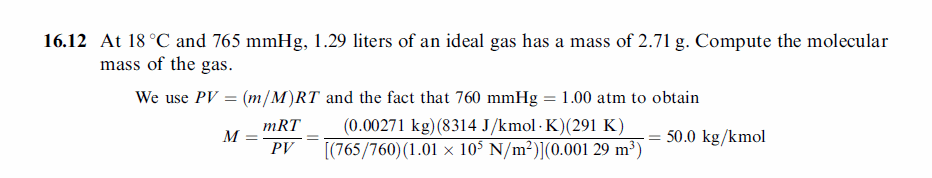
At 18 °C and 765 mmHg, 1.29 liters of an ideal gas has a mass of 2.71 g. Compute the molecular mass of the gas.

PA HELP PO PLSS1. A gas occupies 1.56 L at 770 mmHg. What will be the volume of this gas if the - Brainly.ph

a gas occupies 12.3 litres at a pressure of 40.0 mm HG.what is the volume when the pressure is - Brainly.in
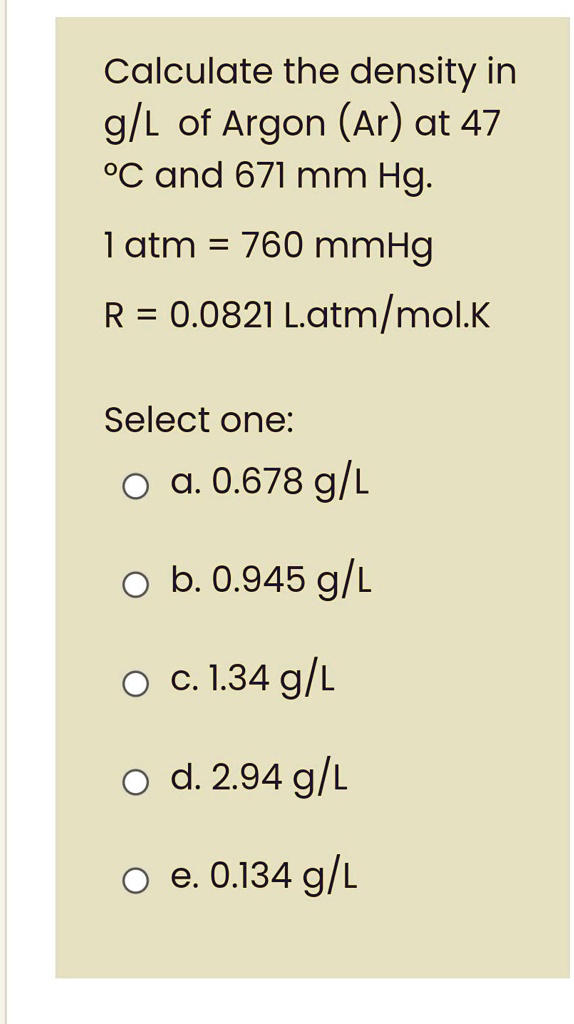
SOLVED:Calculate the density in g/L of Argon (Ar) at 47 'C and 671 mm Hg: Iatm = 760 mmHg R = 0.0821 Latm/mol.k Select one: a. 0.678 g/L b. 0.945 g/L c.1.34
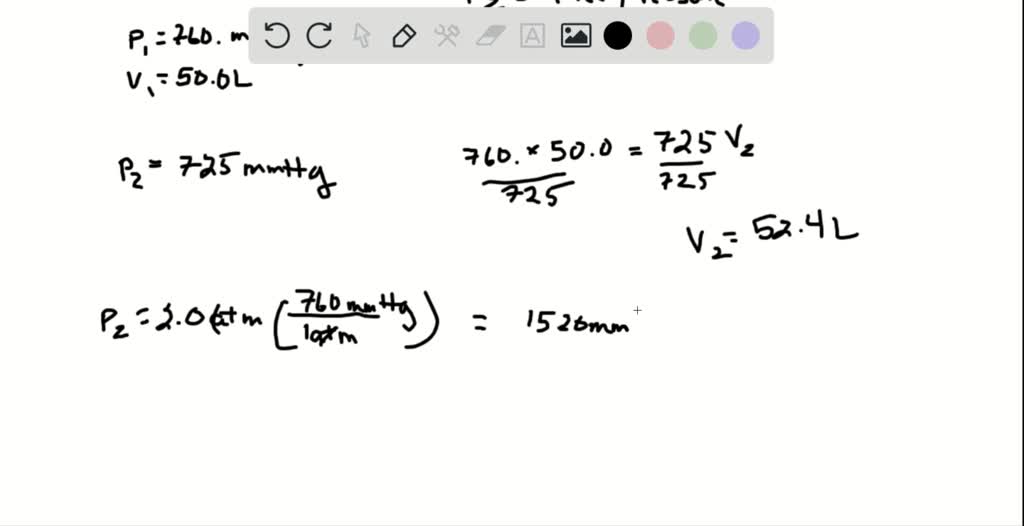
SOLVED:A sample of nitrogen \left(\mathrm{N}_{2}\right) has a volume of 50.0 \mathrm{L} at a pressure of 760 . mmHg. What is the final volume, in liters, of the gas at each of the