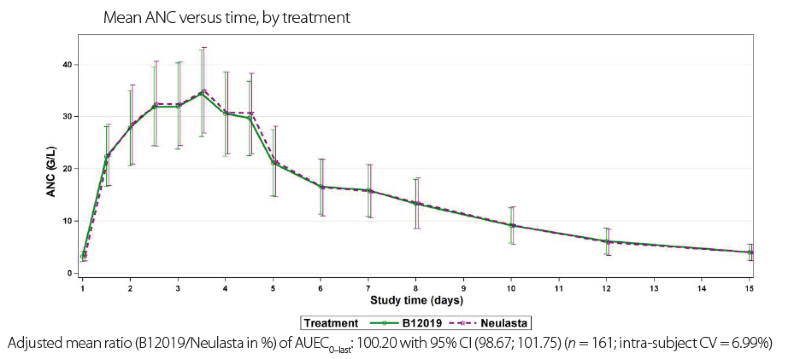
Pharmacokinetics and Pharmacodynamics of a Proposed Pegfilgrastim Biosimilar MSB11455 Versus the Reference Pegfilgrastim Neulasta in Healthy Subjects: A Randomized, Double-blind Trial - Clinical Therapeutics

Neulasta (pegfilgrastim): a once-per-cycle option for the management of chemotherapy-induced neutropenia - ScienceDirect
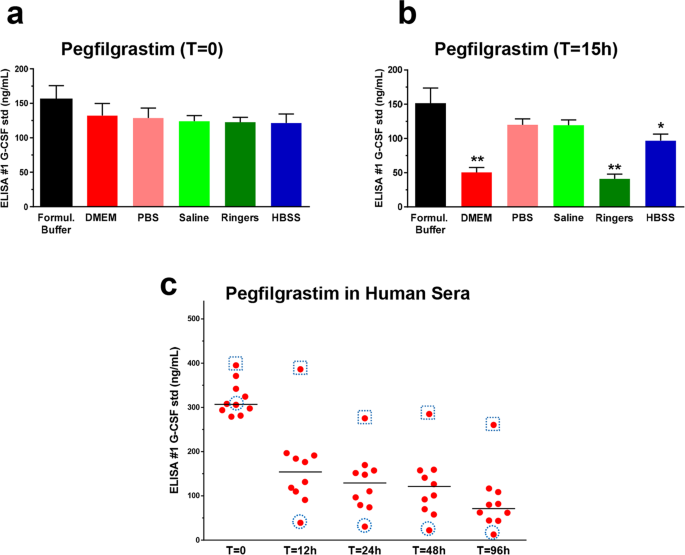
The ELISA Detectability and Potency of Pegfilgrastim Decrease in Physiological Conditions: Key Roles for Aggregation and Individual Variability | Scientific Reports

Comparative costs of Zarzio Õ , Neupogen Õ , and Neulasta Õ associated... | Download Scientific Diagram
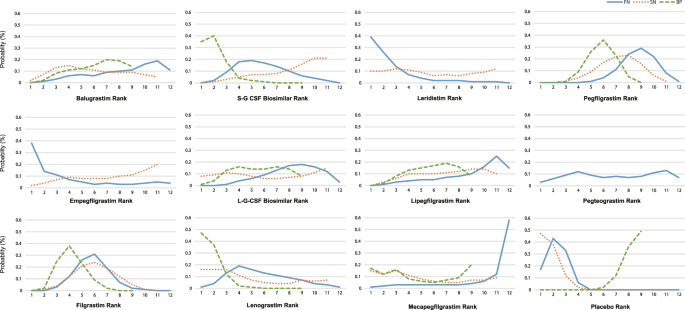
Efficacy and tolerability of granulocyte colony-stimulating factors in cancer patients after chemotherapy: A systematic review and Bayesian network meta-analysis | Scientific Reports
1. NEULASTIM (6 mg in 0.6 mL solution for injection) 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 3. PHARMACEUTICAL FORM 4. CLIN
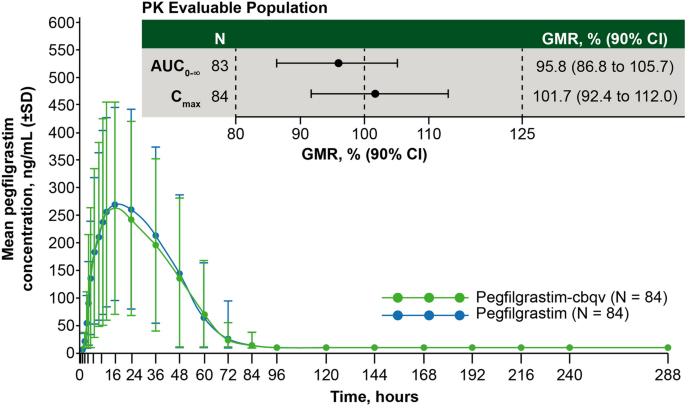
Pharmacokinetic and Pharmacodynamic Equivalence of Pegfilgrastim-cbqv and Pegfilgrastim in Healthy Subjects | SpringerLink
AUSTRALIAN PI - NEULASTA® (PEGFILGRASTIM) 1 NAME OF THE MEDICINE 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 3 PHARMACEUTICAL

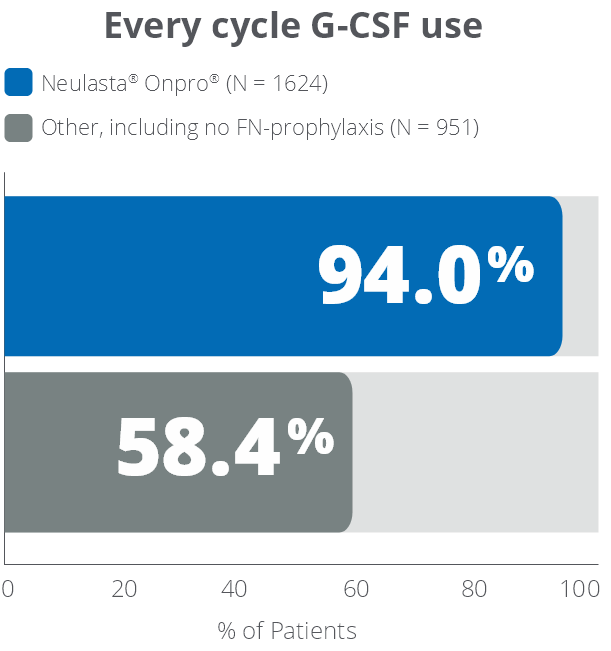
These highlights do not include all the information needed to use NEULASTA safely and effectively. See full prescribing information for NEULASTA. NEULASTA® (pegfilgrastim) injection, for subcutaneous use Initial U.S. Approval: 2002
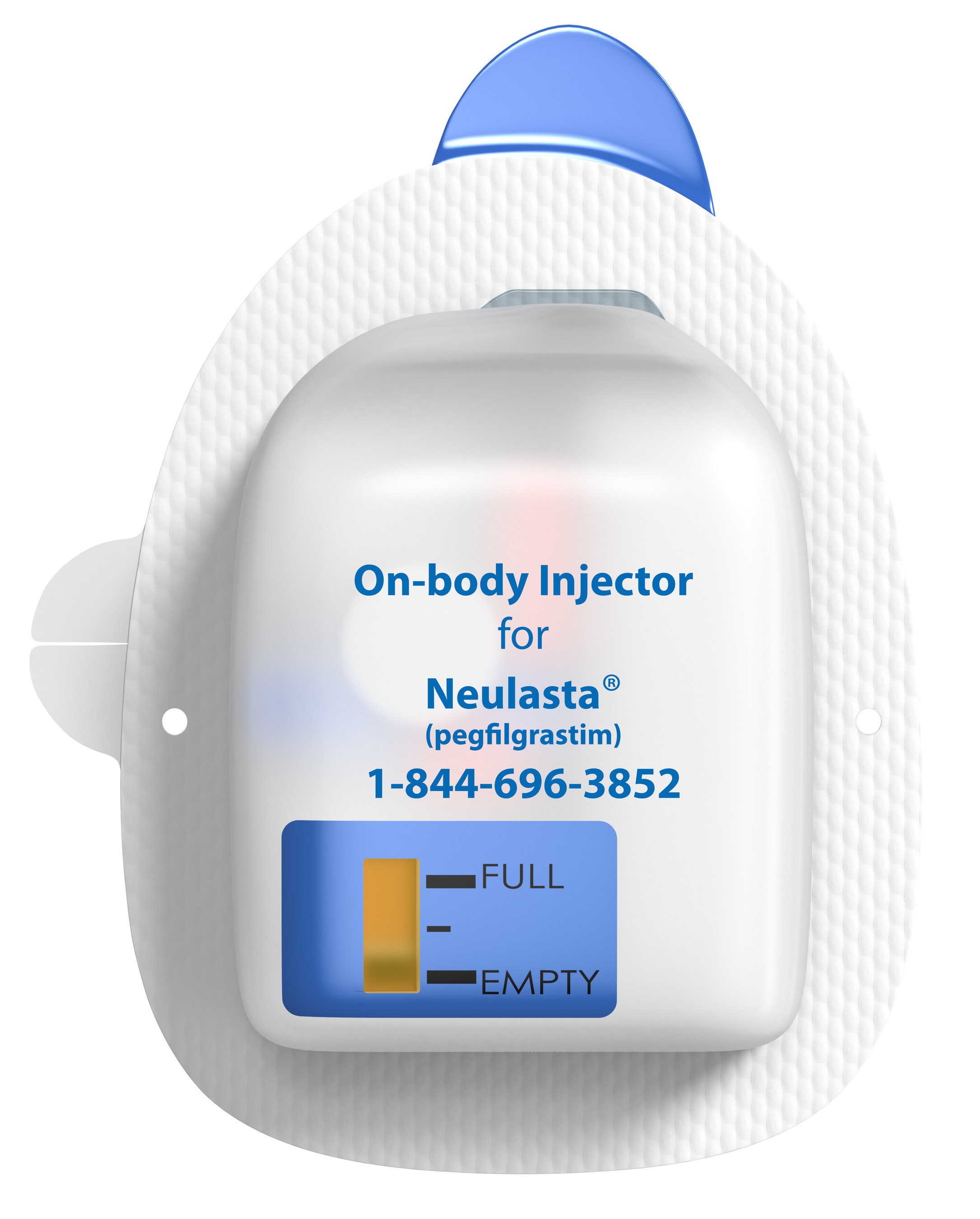
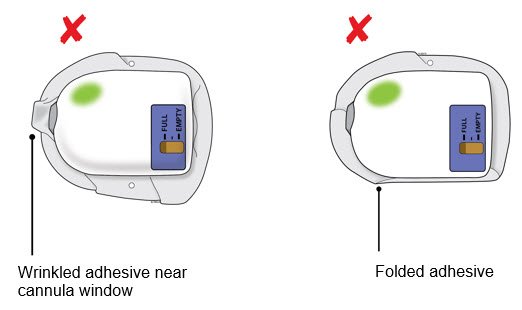
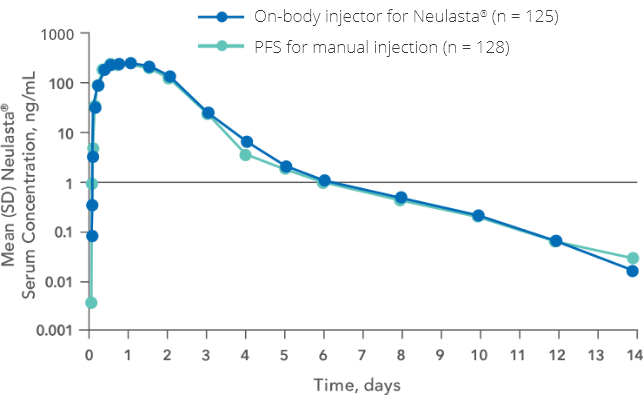
Patient INSTRUCTIONS FOR USE Neulasta® Onpro® (nu-las-tah) (pegfilgrastim) injection Single-Use On-body Injector Your On-body

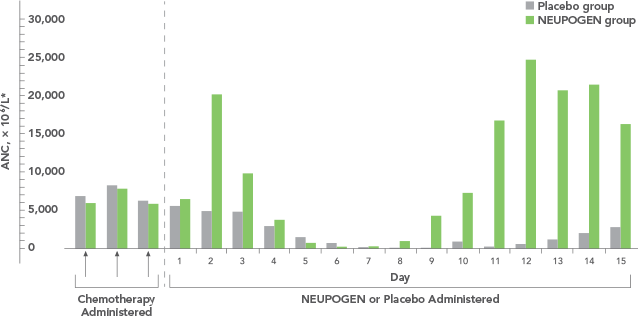
Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim inchemotherapy-induced neutropenia: a multicenterdose-finding study in women with breast cancer - Annals of Oncology